Various Fuels Information Center
페이지 정보

본문
Dimethyl ether (DME) is a synthetically produced alternative to diesel for use in specifically designed compression ignition diesel engines. Under regular atmospheric conditions, DME is a colorless gas. It is used extensively in the chemical business and as an aerosol propellant. Dimethyl ether requires about seventy five pounds per square inch (psi) of strain to be in liquid type. Due to this, DME's dealing with requirements are just like those of propane—both should be saved in pressurized storage tanks at an ambient temperature. The consequences are rapidly reversible which is in step with the very speedy bioelimination of the molecule. DME has, in the past, been thought of for use as a human anesthetic. It ought to be famous that this chemical can produce cardiac sensitization just like the consequences of epinephrine. DME released to the environment would be expected to exist nearly entirely within the vapor section because the vapor stress is 4450 mmHg at 25 ℃. It is vulnerable to photooxidation through vapor phase response with photochemically produced hydroxyl radicals. An atmospheric half-life of 5.4 days has been calculated. It may also exhibit very highmobility in soil and, subsequently, itmay leach to groundwater.
The company's enterprise operations are organized into three important groups: Global Environmental & Infrastructure Enterprise Group, Industrial Finance, Logistics & Improvement Group, and Energy Enterprise Group. Royal Dutch Shell, generally known as Shell, is a British-Dutch multinational oil and gas company. The company is organized into 4 predominant enterprise segments: Upstream, Downstream, Built-in Fuel, and New Energies. The Upstream segment is involved in exploration and production activities, together with conventional and unconventional oil and gas assets. The Downstream phase contains actions equivalent to refining, marketing, and buying and selling of oil and fuel merchandise, as properly because the manufacture and marketing of chemicals. The Integrated Fuel segment is involved within the manufacturing and advertising and marketing of liquefied natural gasoline (LNG) and different gasoline-associated actions. The new Energies phase is concentrated on developing and investing in low-carbon energy sources, similar to wind and solar power.
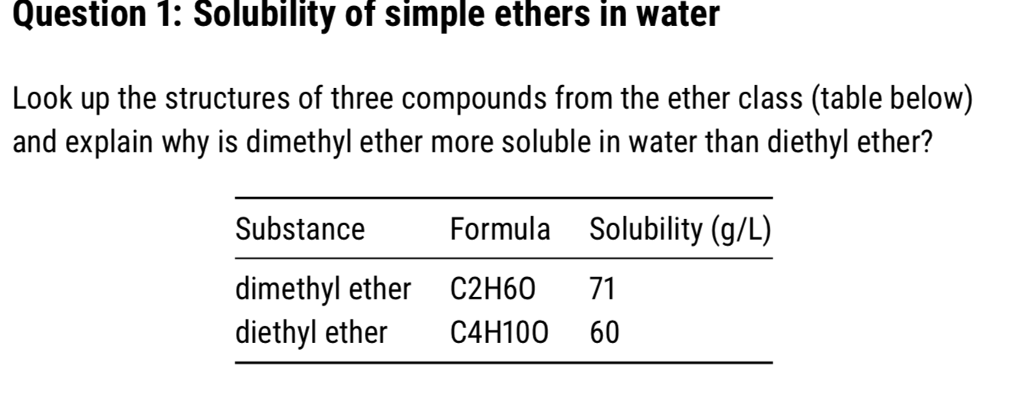
Charged species as a rule dissolve readily in water: in different phrases, they are very hydrophilic (water-loving). Biphenyl, like sodium chloride, is a colorless crystalline substance. Biphenyl doesn't dissolve in any respect in water. It's a very non-polar molecule, with solely carbon-carbon and carbon-hydrogen bonds. It has some intermolecular forces bonding it to itself by way of nonpolar London dispersion forces, but it surely has no important attractive interactions with very polar solvent molecules like water. In the meantime the water molecules themselves are highly linked to each other by hydrogen bonding forces. Included within the FDA Inactive Substances Database (topical aerosols). Included in nonparenteral medicines licensed within the UK. Included in the Canadian Listing of Acceptable Non-medicinal Components. Dimethyl ether, often known as methoxymethane, wood ether, dimethyl oxide or methyl ether, What is the common name for CH3OCH3? the only ether. Dimethyl ether(DME), also referred to as methyl ether and methoxymethane, is the best fatty ether. Dimethyl ether is a colorless compressed liquefied gas or liquid. 27.0%. Hazard Identification(based mostly on NFPA-704 M Ranking System): Health 2,Flammability 4, Reactivity 1. Soluble in water. Dimethyl ether is a liquefied gasoline and exists as a liquid at room temperature when contained below its personal vapor strain, or as a gas when exposed to room temperature and strain. It's a clear, colorless, virtually odorless liquid. In excessive concentrations, the gasoline has a faint ether-like odor.
Exception: Hydrogen tends to realize 2 electrons in its outermost shell to fulfill the He configuration. This is understood as the octet fulfillment rule. If we test the weather within the dimethyl ether molecule, we find that all the hydrogen and carbon atoms have achieved their corresponding required configurations. Nevertheless, oxygen only has 4 valence electrons surrounding it. We've got fulfilled the octet rule and used all the 20 valence electrons. Moreover, DME is gaining momentum as an alternative transportation fuel for diesel owing to its better ignition in compressed engines. It's cleaner, possesses propane-like properties, and has the potential to change diesel in heavy-obligation applications. Robust progress in the gross sales of heavy trucks and automobiles will accelerate the general dimethyl ether market growth in the course of the forecast period.
These electrons kind teams ( the lone pairs and the bonded pairs) and we consider the central atom solely in VSEPR theory for ease of calculation. So, we now have the AX2E2 notation for the methoxymethane molecule. As we can see, we get a bent molecular geometry like water here. The bond angle of C-O-C is round 110 levels on account of methyl groups that experience steric repulsion. The electron geometry is tetrahedral. The highest firms within the dimethyl ether market are investing in creating new applied sciences that can enhance the properties of DME, thus resulting in the development of recent DME-based products with improved performance characteristics. Find out about alternatives, challenges, and tendencies in the global Dimethyl Ether (DME) market with IMARC’s market research report. Dimethyl ether(DME), also known as methyl ether and methoxymethane, is the best fatty ether. It's a derivative of the dehydration condensation of two molecules of methanol. It is colorless, non-toxic, fuel or compressed liquid with slight ether aroma at room temperature. Dimethyl ether has glorious combustion characteristics, high cetane number, low pollution, no damage to the atmospheric ozone layer, and easy degradation in the troposphere. It can be widely used as car gasoline.
- 이전글2024: eulexin goedkoop zonder recept in België 24.11.25
- 다음글Choosing the Perfect Internet Casino 24.11.25
댓글목록
등록된 댓글이 없습니다.